Convert CO2 with renewable energy storage systems
Prototypes can convert CO2 without degrading battery performance
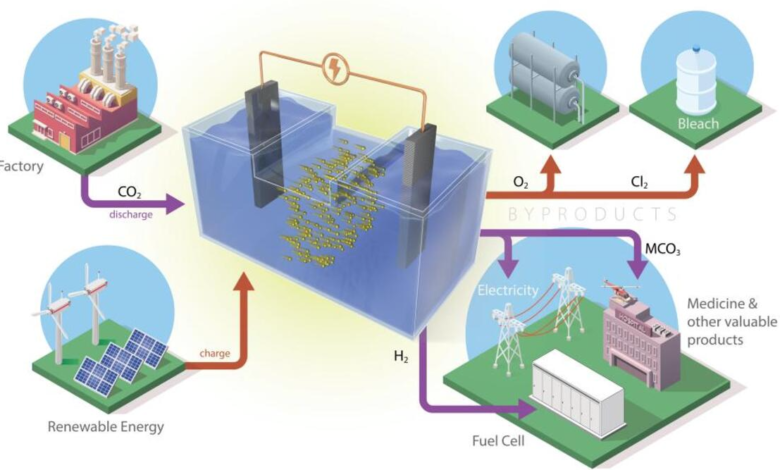
Exploit the electrochemical reaction that occurs during the release of energy from a battery to capture and convert CO2 into products that can be reused. This is the idea behind two new prototypes developed by researchers at the Oak Ridge National Laboratory (ORNL) of the US Doe. Combining storage systems for renewable energy with carbon capture technology.
How does the battery convert CO2?
The basic operation of the two batteries is no different from the traditional ones. The process studied at ORNL uses electrochemical reactions that move the ions between the two electrodes through an electrolyte. In ORNL prototypes, carbon dioxide is added to the liquid electrolyte. During the release of energy from the battery, free electrons are also involved in the process of converting CO2 into a solid powder.
An advantage of this configuration is the possibility of connecting the storage system – for example, a storage system for solar or wind energy – with a source of CO2 captured by industrial activities.
The two prototypes use both abundant materials, bypassing possible criticalities in the supply chain. A model is based on an iron-nickel catalyst and uses sodium in salt water to convert CO2. The other uses aluminium and the same liquid electrolyte.
EU, Simson: strategy for industrial CCS coming soon
One of the crucial steps forward in the applicability of the process concerns converted CO2. In other previous attempts, the solid material resulting from CO2 conversion tends to clog the electrode surface, causing battery performance to degrade. In addition, sodium tends to create a film that reduces or cancels the functionality of the electrodes.
The configuration presented by the ORNL, on the contrary, obtains a by-product that dissolves in liquid electrolytes and improves the performance of the storage system. But it can be filtered – without interrupting the operation of the battery – obtaining a powder that can be further processed and used for industrial uses, for example in the pharmaceutical or cement sectors. In addition, unlike other configurations that release CO2, the cycle only generates oxygen and hydrogen. Which could also be captured and used in other industrial uses.
About the film created by sodium, however, the team of researchers found that irregular discharges during the charging and discharging cycles of the battery are sufficient to reduce or completely avoid its formation.
The ORNL study is available here.





