The rechargeable sector collects a new recipe for electrochemical accumulation
(sustainabilityenvironment.com) – The world of energy storage continues to look for devices that combine high capacity and durability at low cost and high environmental friendliness. Today, new solid state air batteries could open the door to one of the most promising compromises in this direction. Thanks to a group of scientists from the University of Waseda, Japan, who tested a new recipe for the electrochemical cell.
But to understand the degree of innovation we need to take a few steps backwards.
Air batteries, what they are and how they work
Air batteries use environmental oxygen as active material in the positive electrode, metals such as lithium, aluminum or zinc for negative electrodes, and liquid electrolytes. They have attracted the attention of the research thanks to a theoretical specific energy of 11,140 Wh/kg and a maximum capacity of 1100 mAh/g. However at present the most studied devices, such as Li-aria or Zn-air, have inherent problems of high ohmic strength and uncontrolled growth of dendrites, which adversely affect the performance of the battery. In addition to boasting a considerable environmental impact.
This is why in recent years we have tried to replace metals with organic redox-active molecules based on quinone or amine. The result on paper was very satisfactory: an organic air-molecule battery can reach a maximum capacity of over 200 mAh/g for over 60 thousand charge/discharge cycles. In other words, the problems related to the formation of dendrites as well as those of environmental impact are lost. However, the risks related to safety remain, from the high electrical resistance to flammability.
The solid state air batteries
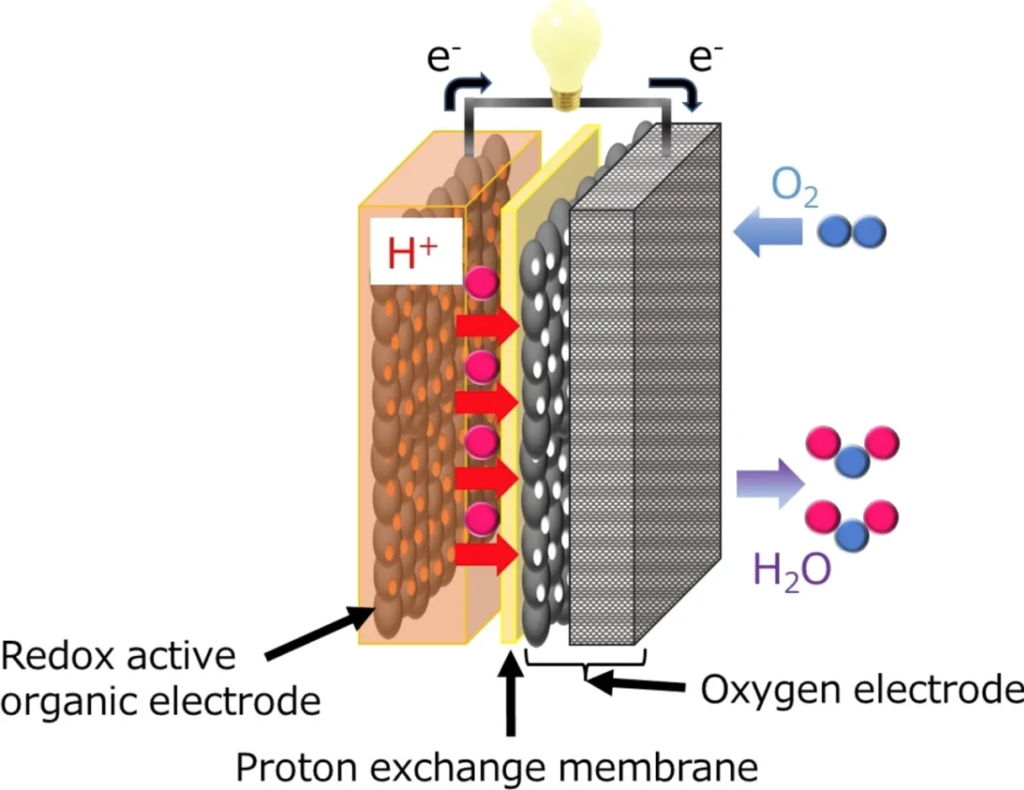
In a recent study published in Angewandte Chemie International Edition, the team led by Professor Kenji Miyatake has developed an innovative solid state air battery (SSAB), evaluating its capacity and durability. Scientists chose a chemical called 2,5-dihydroxy-1,4-benzoquinone (DHBQ) and its polymer PDBM as active materials for the negative electrode due to their stable and reversible redox reactions under acidic conditions. And they used a proton-conducting polymer called Nafion as a solid electrolyte.
“As far as I know, air batteries based on organic electrodes and solid polymer electrolytes have not yet been developed,” Miyatake explained. The team evaluated the charging-discharging performance, speed and cycling characteristics experimentally and found that, unlike typical air-metal batteries, SSAB does not deteriorate in the presence of water and oxygen.
The study is still in its infancy and performance remains low but research has opened a new path for rechargeable batteries. “This technology – said Miyatake – can extend the battery life of small electronic gadgets such as smartphones and contribute to the realization of a carbon-free society”.