How solar green hydrogen can help decarbonize the chemical industry
(sustainabilityenvironment.com) – The simplest and most efficient way to produce green hydrogen is the electrolysis of water: an electrochemical cell, powered by electricity from renewable plants, breaks the molecules of H2O releasing oxygen and hydrogen. The approach in question, however, is not the most direct. There is another technology that can lead to the same result but with fewer steps. Let’s talk about photoelectrolysis, in which cells similar to photovoltaic cells directly use incident light to activate special electrodes immersed in water and promote the same molecular cleavage.
But if today this solution does not receive the same attention as electrolysis on a commercial level, it is for a precise reason: the conversion efficiency is too low. The best photoelectrochemical cells do not exceed 10% in terms of yield. And despite some indisputable advantages – from the low cost of the catalysts used to the possibility of using solar heat to accelerate the reaction – the cost of producing solar green hydrogen seems very uncompetitive. According to data published by the HZB Institute for Solar Fuels, hydrogen produced by photoelectrolysis of water currently costs about 10 dollars per kg. A value double that of “classic” electrolysers and about six times higher than the hydrogen obtained from methane through steam reforming.
A new way to water photoelectrolysis
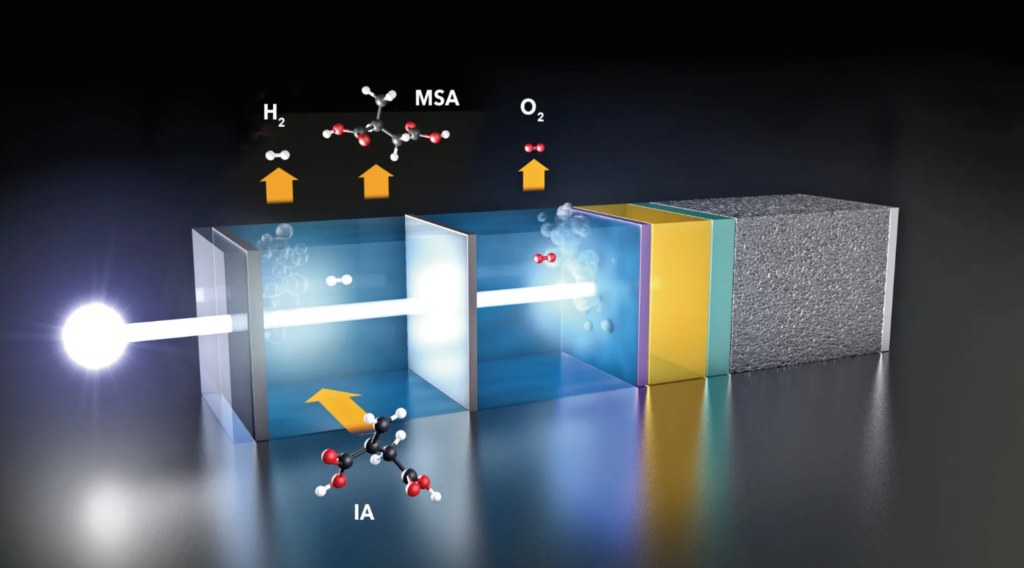
It’s precisely the HZB Institute for Solar Fuels that has found an approach to making green solar hydrogen more competitive. Here a group of scientists studied how equilibrium changes when a portion of the H2 produced with photoelectrochemical cells reacts further in situ with itaconic acid to form methylsuccinic acid (MSA). The latter is a valuable chemical compound whose derivatives are used as solvents in cosmetics, polymer synthesis and pharmaceuticals.
First, the researchers calculated how much energy is needed to produce the cell and how long it must function to reproduce this energy in the form of hydrogen or MSA. For hydrogen alone, the “return time” is about 17 years assuming a modest efficiency of 5%.
Read also A new and extraordinary catalyst for marine hydrogen
If 2% of solar green hydrogen was used to convert itaconic acid to MSA, the return time would be halved. If the percentage increases to 30%, the production energy could be recovered after only 2 years. “This makes the process much more sustainable and competitive,” says Dr Fatwa Abdi. “The system is flexible and can also produce other valuable chemicals”. The results are published in the journal Nature Communications.